PHOENIX — The Delta variant is now responsible for 93% of new cases across the country and is spreading among the younger population.
According to the American Pediatric Academy, there were nearly 72,000 new pediatric cases reported last week. That's an 84% jump from the prior week.
Medical experts continue to stress the importance of COVID-19 vaccines in the fight against the pandemic.
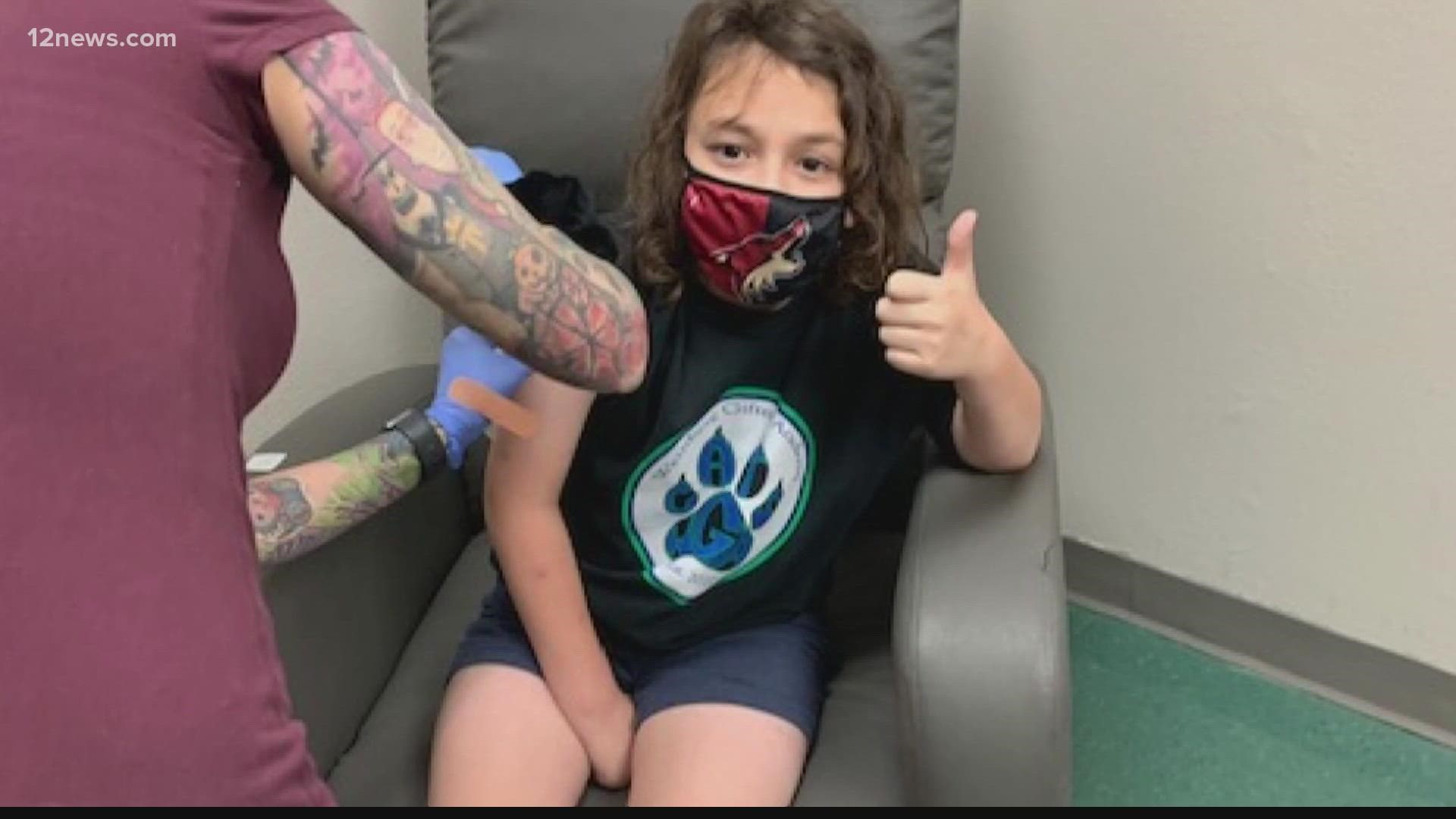
Both Moderna and Pfizer launched vaccine trials for kids between six months and under 12 years old last March.
“We want to make sure the vaccine is safe, especially with our children,” said Pharmetics Moderna Manager of Clinical Operations Jason Wallace.
Wallace is overseeing Moderna's trial in Phoenix, one of eight trial sites in the U.S. The trial is enrolling nearly 7,000 children in groups.
Moderna was the first of the three vaccines authorized for use under the FDA emergency use authorization to begin a study.
“It’s an ever-changing trial. Moderna is being very meticulous and thorough in making sure this is safe and effective for children,” Wallace said.
As of July, 4.2 million children have tested positive for COVID-19. Children and teens represent 19% of cases.
Wallace said there is a high demand from parents who are eager to get kids vaccinated.
“I think we have about 3,000 people right now just on the waiting list and it’s difficult because only a certain number of slots are being at a time because this is happening across the world. They are looking for different demographics,” Wallace said.
Both Moderna and Pfizer are expecting to present results to the Food and Drug Administration sometime this fall, with hopes of a EUA in early to midwinter.
But Wallace said the timeline for Moderna is not certain and safety is the priority.
“We are making sure we get the right dosage that’s going to show the most efficacy for children.”
COVID-19 Vaccine
Subscribe to the 12 News YouTube channel to receive notifications on the latest videos about the coronavirus vaccine and the developments on the distribution in the United States.